Novel immunotherapy with the CDK7 Inhibition Gives New Hope to Small Lung Cancer
As a master regulator of cell cycle progression, cyclin-dependent kinase 7 (CDK7) functions as the catalytic core of CDK-activating kinase (CAK) complex. It is activated upon binding to Cyclin H and Mat1. This trimeric CAK complex activates several CDKs via the phosphorylation, ensuring progression in the cell cycle. Professor Gray’s lab recently reported the novel CDK7 inhibitor, YKL-5-124, potent anti-tumor immunity in small cell lung cancer (SCLC) with the hope of progression towards clinical trial and as new cancer immunotherapy (Figure 1).
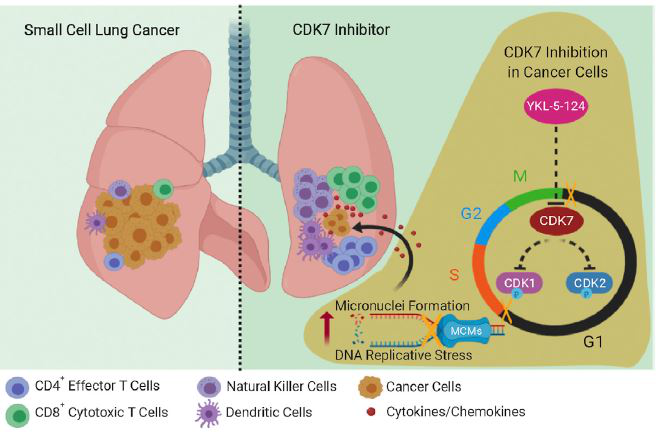
Figure 1: Graphical abstract demonstrating the mechanisms elicited by CDK7 inhibitor, YKL-5-124 in eliciting genomic instability and anti-tumor immunity. (Figure is adopted from the journal article).
YKL-5-124 specifically bound to CDK7 with off-target of CDK12/13. Besides, YKL-5-124 inhibited the CDK1 and CDK2 T-loop phosphorylation. However, YKL-5-124 had no effect on the C-terminal domain (CTD) phosphorylation of RNA Pol ll, suggesting that selective CDK7 inhibition did not inhibit global transcription. These illustrated that YKL-5-124 specifically targeted CDK7. Furthermore, the YKL-5-124 potentiated the cytostatic effects on SCLC cells and implicated cytotoxic effect in longer time points. Besides, cell cycle analysis revealed a G1 phase arrest in SCLC cells upon YKL-5-124 treatment. Besides, the inhibition of CDK7 caused the genome instability an increased in the micronuclei formation. Subsequently, this directed the activation of DNA damage response. Furthermore, YKL-5-124 elicited anti-tumor immunity via the upregulation of pro-inflammatory pathways such as IFNγ and TNFα signalling pathways. These upregulated signalling pathways triggered the release of a vast amount of pro-inflammatory cytokines and chemokines, thus activating the cytotoxic CD8+ T cells.
The initiation of anti-tumor immunity by YKL-5-124 raised up the idea of combination therapy with immune checkpoint inhibitor anti-PD-1. The combination therapy had enhanced the overall survival of tumour-bearing mice. In tumour microenvironment, the mice underwent the treatment had increased CD44 high and CD62L low effector CD4 T cells with high proliferative Ki67 marker. The combination therapy also increased the release of granzyme B by CD8 cytotoxic T cells, led to increased cytotoxic activities in tumor cells. Furthermore, an increase of tumour resident CD11c, CD103 dendritic cells was observed. This assisted CD4 T cells to relay the signal for the activation of CD8 T cells cytolytic activities.
In-depth characterization of the subpopulation of immune cells showed that the combination treatment of YKL-5-124 and anti-PD-1 elevated the natural killer cells and innate lymphoid cells besides T cells. Interestingly, the research team observed that a reduction in the CD4 and CD8 naïve T cells but a dramatic expansion of CD4 and CD8 effector/ memory T cells with upregulation of T cell activation gene signatures, signifying the robust and enhanced anti-tumor immunity.
In summary, the inhibition of CDK7 triggered the strongest the anti-tumor immunity with the co-administration of anti-PD-1 via the modulation of the tumor-immune ecosystem such as elevating the anti-tumoral immune cells. With the further confirmation of clinical trial results, this combination therapy could be a new approach for cancer immunotherapy in treating SCLC patients in future.
The research was published in the Cancer Cell under Cell Press which can be found in https://jitc.biomedcentral.com/articles/10.1186/s40425-019-0785-8#Fig3.
Reference
Zhang H, Christensen CL, Dries R et al. (2020) CDK7 inhibition potentiates genome instability triggering anti-tumor immunity in small cell lung cancer. Cancer Cell. 37: 37-54.

